Synergistic Strategy towards Enhancing Photosynthesizing Reactive Oxygen Species of Covalent Organic Frameworks
Synergistic Strategy towards Enhancing Photosynthesizing Reactive Oxygen Species of Covalent Organic Frameworks
Xiaoning Zhan, Yucheng Jin, Chen Qu, Heyuan Liu, Rong Jiang, Qianjun Zhi, Dongdong Qi, Kang Wang, Bin Han*, Houhe Pan*, Jianzhuang Jiang*
Published Data: 04 Dec 2024
Link: https://onlinelibrary.wiley.com/doi/epdf/10.1002/adfm.202415629
Solar energy is considered as an inexhaustible and sustainable energy source. The catalytic transformations driven by sunlight therefore offer an energy-efficient, environmentally friendly, and promising solution for a wide range of biological and chemical syntheses, in which catalysts that absorb and utilize sunlight have become the focus of research in this field. In the photocatalytic process involving O2 and H2O, the photoinduced electrons and holes generated by catalysts can be immediately converted into a variety of reactive oxygen species (ROSs) including hydroxyl radical (•OH), hydrogen peroxide (H2O2), superoxide anion radical (•O2−), and singlet oxygen (1O2). Typically, photocatalysts for producing ROSs include inorganic materials such as TiO2, CdS, In2S3 and hybrid metal/metal oxide nanostructures, as well as organic materials such as porphyrin, porphin, and phthalocyanine. However, insufficient light absorption, rapid recombination of photogenerated carriers, and lower conversion efficiency of oxygen still largely limit the evolution and practical application of photocatalytic ROSs.
In response to the above issues, Professor Jiang Jianzhuang's team from our department has prepared a series of covalent organic frameworks (COFs) based on porphyrins. Comparative experimental and theoretical studies have revealed that the synergistic interaction between the two building blocks can significantly enhance the yields of ROSs by independently accomplishing photon harvesting and oxygen capture with smooth energy transfer between them. Particularly, the 1O2 yield of 3P-Por-COF was increased to 1.3 and 3.4 times that of the classical PCN-224 and porphyrin molecular aggregates. Furthermore, the TOF of 3P-Por-COF was as high as 271 h−1 with ~100% selectivity under red light irradiation in catalyzing thioanisole to methyl phenyl sulfoxide. The conversion and stability of degradation of toluene gas under natural light were also superior to the conventional Fenton catalytic system. The present results should contribute to the design of high-performance frameworks-based photocatalysts for ROSs production.
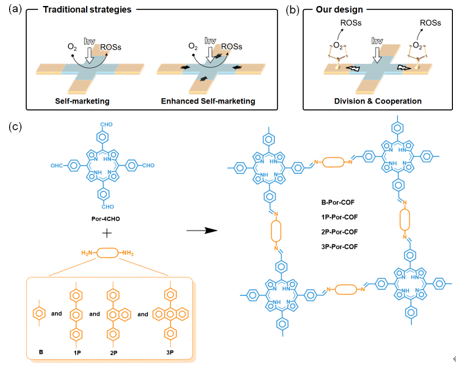
Figure 1. (a) Traditional strategies and (b) Our design for the photosynthesis of ROSs. (c) Schematic synthesis of B-Por-COF, 1P-Por-COF, 2P-Por-COF, and 3P-Por-COF.
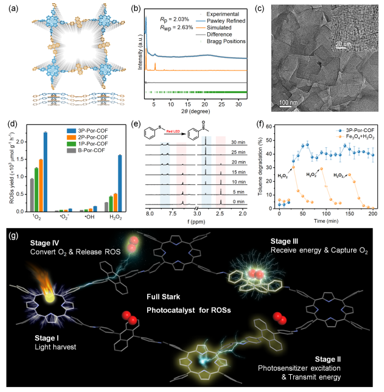
Figure 2 (a) Experimental and simulated PXRD patterns, (b) AA stacking mode in top view and side view, (c) TEM images of 3P-Por-COF. (d) the yield of 1O2, •O2−, •OH, and H2O2 for 3P-Por-COF, (e) Changes in 1H-NMR spectra of catalytic reactions over time (the red shaded area represents the original peak of methylphenyl sulfide, while the blue shaded area represents the newly formed peak of methylphenyl sulfoxide). (f) Time variation and toluene removal efficiencies of the 3P-Por-COF (25 mg) and Fe3O4 (200 mg, added 0.2 mL 30% H2O2 and 0.02 mL 0.1 M H2SO4) under Xenon lamp. (g) Schematic diagram of the mechanism of photocatalytic O2 → ROSs reaction on 3P-Por-COF skeleton.

