Angew. Chem. Int. Ed. (IF: 15.336): Activated Spherical Nucleic Acids for Reversible Protein Capture and Functional Regulation by Research Group of Biochemical Analysis and Molecular Diagnostics, School of Chemistry and Biological Engineering
Abstract: Optical control of protein activity represents a promising strategy for precise modulation of biological processes. Here, we report rationally designed, aptamer-based spherical nucleic acids (SNAs) capable of noninvasive and programmable regulation of target protein activity by deep-tissue-penetrable near-infrared (NIR) light. The photo-responsive SNAs are constructed by integrating activatable aptamer modules onto the surface of upconversion nanoparticles. The SNAs remains inert but can be remotely reverted by NIR light irradiation to capture the target protein and thus function as an enzyme inhibitor, while introduction of antidote DNA could further reverse their inhibition functions. Furthermore, we demonstrate the potential of the SNAs as controllable anticoagulants for the NIR light-triggered regulation of thrombin function. Ultimately, the availability of diverse aptamers would allow the design to regulate the activities of various proteins in a programmable manner.
Recently, Professor Mengyuan Li from the Research Group of Biochemical Analysis and Molecular Diagnostics, School of Chemistry and Biology, has made important research progress in protein capture and functional regulation. Dynamic regulation of protein function is not only crucial for deciphering various biological processes, but will also help to promote protein-related biomedical applications. Traditional methods often require modification of target proteins through genetic engineering or chemical reaction, which is not only complicated and tedious, but also may lead to functional inactivation of the target protein.
To address this challenge, this research group has developed a simple, versatile and non-invasive strategy to achieve the specific capture of protein and reversible modulation of its activity (Figure 1). Near-infrared light-activated spherical nucleic acids (SNAs) were constructed by combining upconversion nanotechnology with DNA aptamer engineering. The recognition capability of the aptamer-modified SNAs towards target protein was inhibited. Upon NIR light irradiation, the functional conformation of DNA aptamer was recovered and thus achieved the specific recognition, capture and function inhibition of the target protein by the SNAs. Importantly, when complementary strands of the aptamer were introduced into the system, the recognition function of the SNA was inhibited again to release the target protein with restored activity, therefore achieving reversible regulation of protein activity. Using thrombin aptamer (a common anticoagulant) as an example, the study demonstrated that the constructed SNAs can specifically capture and reversibly regulate the activity of thrombin in human blood sample. Furthermore, it was verified that the system could be used as a non-invasive regulatory anticoagulant using a mouse thrombosis model. Due to the diversity of aptamers, this strategy can also be extended to the dynamic regulation of other protein functions and provides a new idea for protein-based precise therapy.
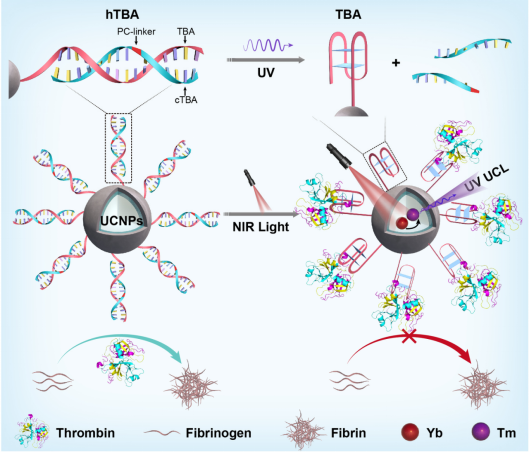
Figure 1. Activatable SNAs for reversible protein capture and dynamic regulation of protein function
This work has been published in Angew. Chem. Int. Ed. (2022, DOI: 10.1002/anie.2027562).) with title "Near-Infrared Light-Activatable Spherical Nucleic Acids for Conditional Control of Protein Activity". Jingfang Zhang, a postdoctoral fellow at the School of Chemistry and Biological Engineering, University of Science and Technology Beijing, is the first author and Professor Mengyuan Li is the corresponding author. The research was supported by the National Natural Science Foundation of China.
Keywords: DNA Aptamers, Protein Capture, Protein Activity Regulation,·Spherical Nucleic Acids, Thrombin
Link to the paper: https://onlinelibrary.wiley.com/doi/10.1002/anie.202117562

