IF: 18.684: Songsong Tang (etc.), Enzyme-powered Janus platelet cell robots for active and targeted drug delivery
IF: 18.684: Songsong Tang (etc.), Enzyme-powered Janus platelet cell robots for active and targeted drug delivery
Songsong Tang, Fangyu Zhang, Hua Gong, Fanan Wei, Jia Zhuang, Emil Karshalev,
Berta Esteban-Fernández de Ávila, Chuying Huang, Zhidong Zhou, Zhengxing Li, Lu Yin,
Haifeng Dong, Ronnie H. Fang, Xueji Zhang*, Liangfang Zhang*, Joseph Wang*. Enzyme-powered Janus platelet cell robots for active and targeted drug delivery. Science Robotics. 5, eaba6137 (2020).
Published Data: 10 Jun 2020
DOI: 10.1126/scirobotics.aba6137
Link: https://robotics.sciencemag.org/content/5/43/eaba6137
ABSTRACT:
Transforming natural cells into functional biocompatible robots capable of active movement is expected to enhance the functions of the cells and revolutionize the development of synthetic micromotors. However, present cell-based micromotor systems commonly require the propulsion capabilities of rigid motors, external fields, or harsh conditions, which may compromise biocompatibility and require complex actuation equipment. Here, we report on an endogenous enzyme-powered Janus platelet micromotor (JPL-motor) system prepared by immobilizing urease asymmetrically onto the surface of natural platelet cells. This Janus distribution of urease on platelet cells enables uneven decomposition of urea in biofluids to generate enhanced chemophoretic motion. The cell surface engineering with urease has negligible impact on the functional surface proteins of platelets, and hence, the resulting JPL-motors preserve the intrinsic biofunctionalities of platelets, including effective targeting of cancer cells and bacteria. The efficient propulsion of JPL-motors in the presence of the urea fuel greatly enhances their binding efficiency with these biological targets and improves their therapeutic efficacy when loaded with model anticancer or antibiotic drugs. Overall, asymmetric enzyme immobilization on the platelet surface leads to a biogenic microrobotic system capable of autonomous movement using biological fuel. The ability to impart self-propulsion onto biological cells, such as platelets, and to load these cellular robots with a variety of functional components holds considerable promise for developing multifunctional cell-based micromotors for a variety of biomedical applications.
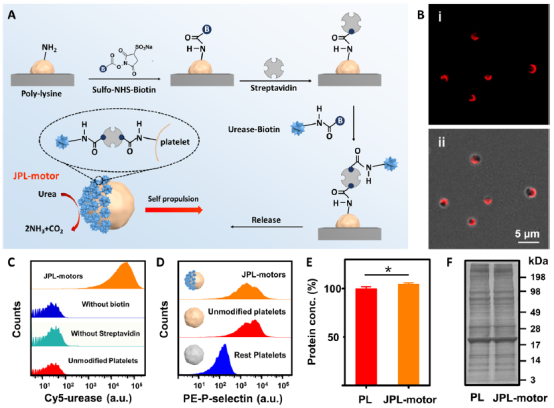
Figure 1. Fabrication and characterization of JPL-motors. (A) Schematic of the fabrication of JPL-motors. (B) Fluorescent (i) and merged (ii) images of Cy5 and bright-field channels of JPL-motors with Cy5-labeled urease. (C) Representative flow cytometry histograms of Cy5-labeled urease under different conditions: JPL-motors (orange) and control experiments with unmodified platelets (red) and Janus modification of platelets without using biotin on urease (blue) or streptavidin (cyan). (D) Representative flow cytometry histograms of the platelet activation marker P-selectin on JPL-motors (orange), unmodified platelets (red), and resting platelets (blue). (E) Quantification of the protein content of unmodified platelets (PL; red) and JPL-motors (orange) (both 5 × 1011 cells/ml), stored in 1× PBS at 4°C for 24 hours. The mean protein amount of unmodified platelets was normalized to 100% (*P < 0.05, t test). (F) SDS–polyacrylamide gel electrophoresis analysis of proteins presented on unmodified platelets (PL) and JPL-motors. The samples were run at equal protein content and stained with Coomassie blue.
Figure 2. The “carousel” of images on the home page of Science Robotics.

